Swine influenza
Swine influenza, also called pig influenza, swine flu, hog flu and pig flu, is an infection by any one of several types of swine influenza virus. Swine influenza virus (SIV) or S-OIV (swine-origin influenza virus) is any strain of the influenza family of viruses that is endemic in pigs.[2] As of 2009, the known SIV strains include influenza C and the subtypes of influenza A known as H1N1, H1N2, H3N1,H3N2, and H2N3.
Swine influenza virus is common throughout pig populations worldwide. Transmission of the virus from pigs to humans is not common and does not always lead to human flu, often resulting only in the production of antibodies in the blood. If transmission does cause human flu, it is called zoonotic swine flu. People with regular exposure to pigs are at increased risk of swine flu infection. The meat of an infected animal poses no risk of infection when properly cooked.
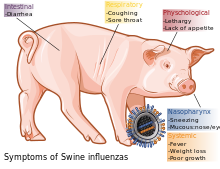
During the mid-20th century, identification of influenza subtypes became possible, allowing accurate diagnosis of transmission to humans. Since then, only 50 such transmissions have been confirmed. These strains of swine flu rarely pass from human to human. Symptoms of zoonotic swine flu in humans are similar to those of influenza and of influenza-like illness in general, namely chills, fever, sore throat,muscle pains, severe headache, coughing, weakness and general discomfort.
Contents |
[edit]Classification
Of the three genera of influenza viruses that cause human flu, two also cause influenza in pigs, with influenza A being common in pigs andinfluenza C being rare.[4] Influenza B has not been reported in pigs. Within influenza A and influenza C, the strains found in pigs and humans are largely distinct, although because of reassortment there have been transfers of genes among strains crossing swine, avian, and human species boundaries.
[edit]Influenza C
Influenza C viruses infect both humans and pigs, but do not infect birds.[5] Transmission between pigs and humans have occurred in the past.[6] For example, influenza C caused small outbreaks of a mild form of influenza amongst children in Japan[7] and California.[7] Because of its limited host range and the lack of genetic diversity in influenza C, this form of influenza does not cause pandemics in humans.[8]
[edit]Influenza A
Swine influenza is known to be caused by influenza A subtypes H1N1,[9] H1N2,[9] H2N3,[10] H3N1,[11] and H3N2.[9] In pigs, three influenza A virus subtypes (H1N1, H1N2, and H3N2) are the most common strains worldwide.[12] In the United States, the H1N1 subtype was exclusively prevalent among swine populations before 1998; however, since late August 1998, H3N2 subtypes have been isolated from pigs. As of 2004, H3N2 virus isolates in US swine and turkey stocks were triple reassortants, containing genes from human (HA, NA, and PB1), swine (NS, NP, and M), and avian (PB2 and PA) lineages.[13]
[edit]Surveillance
Although there is no formal national surveillance system in the United States to determine what viruses are circulating in pigs,[14] there is an informal surveillance network in the United States that is part of a world surveillance network.
Veterinary medical pathologist, Tracey McNamara, set up a national disease surveillance system in zoos because the zoos do active disease surveillance and many of the exotic animals housed there have broad susceptibilities. Many species fall below the radar of any federal agencies (including dogs, cats, pet prairie dogs, zoo animals, and urban wildlife), even though they may be important in the early detection of human disease outbreaks.[15] [16]
[edit]History
Swine influenza was first proposed to be a disease related to human flu during the 1918 flu pandemic, when pigs became sick at the same time as humans.[17] The first identification of an influenza virus as a cause of disease in pigs occurred about ten years later, in 1930.[18] For the following 60 years, swine influenza strains were almost exclusively H1N1. Then, between 1997 and 2002, new strains of three different subtypes and five different genotypes emerged as causes of influenza among pigs in North America. In 1997–1998, H3N2 strains emerged. These strains, which include genes derived by reassortment from human, swine and avian viruses, have become a major cause of swine influenza in North America. Reassortment between H1N1 and H3N2 produced H1N2. In 1999 in Canada, a strain of H4N6 crossed the species barrier from birds to pigs, but was contained on a single farm.[18]
The H1N1 form of swine flu is one of the descendants of the strain that caused the 1918 flu pandemic.[19][20] As well as persisting in pigs, the descendants of the 1918 virus have also circulated in humans through the 20th century, contributing to the normal seasonal epidemics of influenza.[20] However, direct transmission from pigs to humans is rare, with only 12 recorded cases in the U.S. since 2005.[21] Nevertheless, the retention of influenza strains in pigs after these strains have disappeared from the human population might make pigs a reservoir where influenza viruses could persist, later emerging to reinfect humans once human immunity to these strains has waned.[22]
Swine flu has been reported numerous times as a zoonosis in humans, usually with limited distribution, rarely with a widespread distribution. Outbreaks in swine are common and cause significant economic losses in industry, primarily by causing stunting and extended time to market. For example, this disease costs the British meat industry about £65 million every year.[23]
[edit]1918 pandemic in humans
The 1918 flu pandemic in humans was associated with H1N1 and influenza appearing in pigs;[20] this may reflect a zoonosis either from swine to humans, or from humans to swine. Although it is not certain in which direction the virus was transferred, some evidence suggests that, in this case, pigs caught the disease from humans.[17] For instance, swine influenza was only noted as a new disease of pigs in 1918, after the first large outbreaks of influenza amongst people.[17] Although a recent phylogenetic analysis of more recent strains of influenza in humans, birds, and swine suggests that the 1918 outbreak in humans followed a reassortment event within a mammal,[24] the exact origin of the 1918 strain remains elusive.[25] It is estimated that anywhere from 50 to 100 million people were killed worldwide.[20][26]
[edit]1976 U.S. outbreak
Main article: 1976 swine flu outbreak
On February 5, 1976, in the United States an army recruit at Fort Dix said he felt tired and weak. He died the next day and four of his fellow soldiers were later hospitalized. Two weeks after his death, health officials announced that the cause of death was a new strain of swine flu. The strain, a variant of H1N1, is known as A/New Jersey/1976 (H1N1). It was detected only from January 19 to February 9 and did not spread beyond Fort Dix.[27]
This new strain appeared to be closely related to the strain involved in the 1918 flu pandemic. Moreover, the ensuing increased surveillance uncovered another strain in circulation in the U.S.:A/Victoria/75 (H3N2) spread simultaneously, also caused illness, and persisted until March.[27]Alarmed public-health officials decided action must be taken to head off another major pandemic, and urged President Gerald Ford that every person in the U.S. be vaccinated for the disease.[28]
The vaccination program was plagued by delays and public relations problems.[29] On October 1, 1976, immunizations began and three senior citizens died soon after receiving their injections. This resulted in a media outcry that linked these deaths to the immunizations, despite the lack of any proof that the vaccine was the cause. According to science writer Patrick Di Justo, however, by the time the truth was known—that the deaths were not proven to be related to the vaccine—it was too late. "The government had long feared mass panic about swine flu—now they feared mass panic about the swine flu vaccinations." This became a strong setback to the program.[30]
There were reports of Guillain-Barré syndrome, a paralyzing neuromuscular disorder, affecting some people who had received swine flu immunizations. Although if a link exists is still not clear, this syndrome may be a rare side-effect of influenza vaccines. As a result, Di Justo writes that "the public refused to trust a government-operated health program that killed old people and crippled young people." In total, 48,161,019 Americans, or just over 22% of the population, had been immunized by the time the National Influenza Immunization Program(NIIP) was effectively halted on December 16, 1976.[31] [32]
Overall, there were 1098 cases of Guillain-Barré Syndrome (GBS) recorded nationwide by CDC surveillance, 532 of which occurred after vaccination and 543 before vaccination.[33] There are about one to two cases of GBS per 100,000 people every year, whether or not people have been vaccinated.[34] The vaccination program seems to have increased this normal risk of developing GBS by about to one extra case per 100,000 vaccinations.[34]
The CDC states that most studies on modern influenza vaccines have seen no link with GBS,[34][35][36] Although one review gives an incidence of about one case per million vaccinations,[37] a large study in China, reported in the NEJM covering close to 100 million doses of H1N1 flu vaccine found only eleven cases of Guillain-Barre syndrome, which is lower than the normal rate of the disease in China; "The risk-benefit ratio, which is what vaccines and everything in medicine is about, is overwhelmingly in favor of vaccination."[38]
[edit]1988 zoonosis
In September 1988, a swine flu virus killed one woman and infected others. 32-year old Barbara Ann Wieners was eight months pregnant when she and her husband, Ed, became ill after visiting the hog barn at a county fair in Walworth County, Wisconsin. Barbara died eight days later, after developing pneumonia.[39] The only pathogen identified was an H1N1 strain of swine influenza virus.[40] Doctors were able to induce labor and deliver a healthy daughter before she died. Her husband recovered from his symptoms.
Influenza-like illness (ILI) was reportedly widespread among the pigs exhibited at the fair. Of the 25 swine exhibitors aged 9 to 19 at the fair, 19 tested positive for antibodies to SIV, but no serious illnesses were seen. The virus was able to spread between people, since 1-3 health care personnel who had cared for the pregnant woman developed mild influenza-like illnesses, and antibody tests suggested that they had been infected with swine flu. However, there was no community outbreak.[41][42]
[edit]1998 US outbreak in swine
In 1998, swine flu was found in pigs in four U.S. states. Within a year, it had spread through pig populations across the United States. Scientists found that this virus had originated in pigs as a recombinant form of flu strains from birds and humans. This outbreak confirmed that pigs can serve as a crucible where novel influenza viruses emerge as a result of the reassortment of genes from different strains.[43][44][45] Genetic components of these 1998 triple-hybrid stains would later form six out of the eight viral gene segments in the 2009 flu outbreak.[46][47][48][49][50]
[edit]2007 Philippine outbreak in swine
| This section requires expansion. |
On August 20, 2007, the Department of Agriculture officers investigated the outbreak (epizootic) of swine flu in Nueva Ecija and CentralLuzon, Philippines. The mortality rate is less than 10% for swine flu, unless there are complications like hog cholera. On July 27, 2007, the Philippine National Meat Inspection Service (NMIS) raised a hog cholera "red alert" warning over Metro Manila and 5 regions of Luzon after the disease spread to backyard pig farms in Bulacan and Pampanga, even if these tested negative for the swine flu virus.[51][52]
[edit]2010 Northern Ireland outbreak in swine
Since November 2010, there have been 14 reported deaths as a result of swine flu in Northern Ireland. The majority of the victims were reported to have pre-existing health conditions which had lowered the patients' immune system. This closely corresponds to the 19 patients that had died in the year prior due to swine flu where 18 of the 19 were determined to have lowered immune systems. Because of this, many mothers whom have just given birth are strongly encouraged to get a flu shot because their immune systems are vulnerable. Also, studies have shown that people between the ages of 15 and 44 have the highest rate of infection. Although most people now recover, having any conditions that lower ones immune system increases the risk of having the flu become potentially lethal. In Northern Ireland now, approximately 56% of all people under 65 that are entitled to the vaccine have gotten the shot and the outbreak is said to be under control.[53]
[edit]Transmission
[edit]Transmission between pigs
Influenza is quite common in pigs, with about half of breeding pigs having been exposed to the virus in the US.[54] Antibodies to the virus are also common in pigs in other countries.[54]
The main route of transmission is through direct contact between infected and uninfected animals.[12] These close contacts are particularly common during animal transport. Intensive farming may also increase the risk of transmission, as the pigs are raised in very close proximity to each other.[55][56] The direct transfer of the virus probably occurs either by pigs touching noses, or through dried mucus. Airborne transmission through the aerosols produced by pigs coughing or sneezing are also an important means of infection.[12] The virus usually spreads quickly through a herd, infecting all the pigs within just a few days.[2] Transmission may also occur through wild animals, such aswild boar, which can spread the disease between farms.[57]
[edit]Transmission to humans
People who work with poultry and swine, especially people with intense exposures, are at increased risk of zoonotic infection with influenza virus endemic in these animals, and constitute a population of human hosts in which zoonosis and reassortment can co-occur.[58]Vaccination of these workers against influenza and surveillance for new influenza strains among this population may therefore be an important public health measure.[59] Transmission of influenza from swine to humans who work with swine was documented in a small surveillance study performed in 2004 at the University of Iowa.[60] This study among others forms the basis of a recommendation that people whose jobs involve handling poultry and swine be the focus of increased public health surveillance.[58] Other professions at particular risk of infection are veterinarians and meat processing workers, although the risk of infection for both of these groups is lower than that of farm workers.[61]
[edit]Interaction with avian H5N1 in pigs
Pigs are unusual as they can be infected with influenza strains that usually infect three different species: pigs, birds and humans.[62] This makes pigs a host where influenza viruses might exchange genes, producing new and dangerous strains.[62] Avian influenza virus H3N2 isendemic in pigs in China and has been detected in pigs in Vietnam, increasing fears of the emergence of new variant strains.[63] H3N2evolved from H2N2 by antigenic shift.[64] In August 2004, researchers in China found H5N1 in pigs.[65]
These H5N1 infections may be quite common: in a survey of 10 apparently healthy pigs housed near poultry farms in West Java, where avian flu had broken out, five of the pig samples contained the H5N1 virus. The Indonesian government has since found similar results in the same region. Additional tests of 150 pigs outside the area were negative.[66][67]
[edit]Signs and symptoms
[edit]In swine
In pigs influenza infection produces fever, lethargy, sneezing, coughing, difficulty breathing and decreased appetite.[12] In some cases the infection can cause abortion. Although mortality is usually low (around 1–4%),[2] the virus can produce weight loss and poor growth, causing economic loss to farmers.[12] Infected pigs can lose up to 12 pounds of body weight over a 3 to 4 week period.[12]
[edit]In humans
Direct transmission of a swine flu virus from pigs to humans is occasionally possible (calledzoonotic swine flu). In all, 50 cases are known to have occurred since the first report in medical literature in 1958, which have resulted in a total of six deaths.[69] Of these six people, one was pregnant, one had leukemia, one had Hodgkin's lymphoma and two were known to be previously healthy.[69] Despite these apparently low numbers of infections, the true rate of infection may be higher, since most cases only cause a very mild disease, and will probably never be reported or diagnosed.[69]
According to the Centers for Disease Control and Prevention (CDC), in humans the symptoms of the 2009 "swine flu" H1N1 virus are similar to those of influenza and of influenza-like illness in general. Symptoms include fever, cough, sore throat, body aches, headache, chills and fatigue. The 2009 outbreak has shown an increased percentage of patients reporting diarrhea andvomiting.[71] The 2009 H1N1 virus is not zoonotic swine flu, as it is not transmitted from pigs to humans, but from person to person.
Because these symptoms are not specific to swine flu, a differential diagnosis of probable swine flu requires not only symptoms but also a high likelihood of swine flu due to the person's recent history. For example, during the 2009 swine flu outbreak in the United States, CDC advised physicians to "consider swine influenza infection in the differential diagnosis of patients with acute febrile respiratory illness who have either been in contact with persons with confirmed swine flu, or who were in one of the five U.S. states that have reported swine flu cases or in Mexico during the 7 days preceding their illness onset."[72] A diagnosis of confirmed swine flu requires laboratory testing of a respiratory sample (a simple nose and throat swab).[72]
The most common cause of death is respiratory failure. Other causes of death are pneumonia(leading to sepsis),[73] high fever (leading to neurological problems), dehydration (from excessive vomiting and diarrhea), electrolyte imbalance and kidney failure.[74] Fatalities are more likely in young children and the elderly.
[edit]Diagnosis
The CDC recommends real time RT-PCR as the method of choice for diagnosing H1N1.[75] This method allows a specific diagnosis of novel influenza (H1N1) as opposed to seasonal influenza. Near-patient point of care tests are in development.[76]
[edit]Prevention
Prevention of swine influenza has three components: prevention in swine, prevention of transmission to humans, and prevention of its spread among humans.
[edit]In swine
Methods of preventing the spread of influenza among swine include facility management, herd management, and vaccination (ATCvet code: QI09AA03). Because much of the illness and death associated with swine flu involves secondary infection by other pathogens, control strategies that rely on vaccination may be insufficient.
Control of swine influenza by vaccination has become more difficult in recent decades, as theevolution of the virus has resulted in inconsistent responses to traditional vaccines. Standard commercial swine flu vaccines are effective in controlling the infection when the virus strains match enough to have significant cross-protection, and custom (autogenous) vaccines made from the specific viruses isolated are created and used in the more difficult cases.[77][78] Presentvaccination strategies for SIV control and prevention in swine farms typically include the use of one of several bivalent SIV vaccines commercially available in the United States. Of the 97 recent H3N2 isolates examined, only 41 isolates had strong serologic cross-reactions with antiserum to three commercial SIV vaccines. Since the protective ability of influenza vaccines depends primarily on the closeness of the match between the vaccine virus and the epidemic virus, the presence of nonreactive H3N2 SIV variants suggests that current commercial vaccines might not effectively protect pigs from infection with a majority of H3N2 viruses.[69][79] The United States Department of Agriculture researchers say that while pig vaccination keeps pigs from getting sick, it does not block infection or shedding of the virus.[80]
Facility management includes using disinfectants and ambient temperature to control virus in the environment. The virus is unlikely to survive outside living cells for more than two weeks, except in cold (but above freezing) conditions, and it is readily inactivated by disinfectants.[2]Herd management includes not adding pigs carrying influenza to herds that have not been exposed to the virus. The virus survives in healthy carrier pigs for up to 3 months and can be recovered from them between outbreaks. Carrier pigs are usually responsible for the introduction of SIV into previously uninfected herds and countries, so new animals should be quarantined.[54] After an outbreak, as immunity in exposed pigs wanes, new outbreaks of the same strain can occur.[2]
[edit]In humans
- Prevention of pig to human transmission
Swine can be infected by both avian and human flu strains of influenza, and therefore are hosts where the antigenic shifts can occur that create new influenza strains.
The transmission from swine to human is believed to occur mainly in swine farms where farmers are in close contact with live pigs. Although strains of swine influenza are usually not able to infect humans this may occasionally happen, so farmers and veterinarians are encouraged to use a facemask when dealing with infected animals. The use of vaccines on swine to prevent their infection is a major method of limiting swine to human transmission. Risk factors that may contribute to swine-to-human transmission include smoking and, especially, not wearing gloves when working with sick animals—thereby increasing the likelihood of subsequent hand-to-eye, hand-to-nose or hand-to-mouth transmission.[81]
- Prevention of human to human transmission
Influenza spreads between humans when infected people cough or sneeze, then other people breathe in the virus or touch something with the virus on it and then touch their own face.[82] "Avoid touching your eyes, nose or mouth. Germs spread this way."[83] Swine flu cannot be spread by pork products, since the virus is not transmitted through food.[82] The swine flu in humans is most contagious during the first five days of the illness although some people, most commonly children, can remain contagious for up to ten days. Diagnosis can be made by sending a specimen, collected during the first five days for analysis.[84]
Recommendations to prevent spread of the virus among humans include using standard infection control against influenza. This includes frequent washing of hands with soap and water or withalcohol-based hand sanitizers, especially after being out in public.[85] Chance of transmission is also reduced by disinfecting household surfaces, which can be done effectively with a diluted chlorine bleach solution.[86]
Experts agree that hand-washing can help prevent viral infections, including ordinary influenza and the swine flu virus. Also not touching your eyes, nose or mouth with your hands helps to prevent the flu.[83] Influenza can spread in coughs or sneezes, but an increasing body of evidence shows small droplets containing the virus can linger on tabletops, telephones and other surfaces and be transferred via the fingers to the eyes, nose or mouth. Alcohol-based gel or foam hand sanitizerswork well to destroy viruses and bacteria. Anyone with flu-like symptoms such as a sudden fever, cough or muscle aches should stay away from work or public transportation and should contact a doctor for advice.[87]
Social distancing is another tactic. It means staying away from other people who might be infected and can include avoiding large gatherings, spreading out a little at work, or perhaps staying home and lying low if an infection is spreading in a community. Public health and other responsible authorities have action plans which may request or require social distancing actions depending on the severity of the outbreak.
[edit]Vaccination
Main article: 2009 flu pandemic vaccine
Vaccines are available for different kinds of swine flu. The U.S. Food and Drug Administration (FDA) approved the new swine flu vaccine for use in the United States on September 15, 2009.[88] Studies by the National Institutes of Health (NIH), show that a single dose creates enough antibodies to protect against the virus within about 10 days.[89]
[edit]Treatment
[edit]In swine
As swine influenza is rarely fatal to pigs, little treatment beyond rest and supportive care is required.[54] Instead veterinary efforts are focused on preventing the spread of the virus throughout the farm, or to other farms.[12] Vaccination and animal management techniques are most important in these efforts. Antibiotics are also used to treat this disease, which although they have no effect against the influenza virus, do help prevent bacterial pneumonia and other secondary infections in influenza-weakened herds.[54]
[edit]In humans
If a person becomes sick with swine flu, antiviral drugs can make the illness milder and make the patient feel better faster. They may also prevent serious flu complications. For treatment, antiviral drugs work best if started soon after getting sick (within 2 days of symptoms). Beside antivirals, supportive care at home or in hospital, focuses on controlling fevers, relieving pain and maintaining fluid balance, as well as identifying and treating any secondary infections or other medical problems. The U.S. Centers for Disease Control and Preventionrecommends the use of Tamiflu (oseltamivir) or Relenza (zanamivir) for the treatment and/or prevention of infection with swine influenza viruses; however, the majority of people infected with the virus make a full recovery without requiring medical attention or antiviral drugs.[90]The virus isolates in the 2009 outbreak have been found resistant to amantadine and rimantadine.[91]
In the U.S., on April 27, 2009, the FDA issued Emergency Use Authorizations to make available Relenza and Tamiflu antiviral drugs to treat the swine influenza virus in cases for which they are currently unapproved. The agency issued these EUAs to allow treatment of patients younger than the current approval allows and to allow the widespread distribution of the drugs, including by non-licensed volunteers.[92]
[edit]See also
[edit]Notes
- ^ International Committee on Taxonomy of Viruses. "The Universal Virus Database, version 4: Influenza A".[dead link]
- ^ a b c d e f "Swine influenza". The Merck Veterinary Manual. 2008. ISBN 1442167424. Retrieved April 30, 2009.
- ^ [1][dead link]
- ^ Heinen PP (15 September 2003). "Swine influenza: a zoonosis". Veterinary Sciences Tomorrow. ISSN 1569-0830. "Influenza B and C viruses are almost exclusively isolated from man, although influenza C virus has also been isolated from pigs and influenza B has recently been isolated from seals.".
- ^ Bouvier NM, Palese P (September 2008). "The biology of influenza viruses". Vaccine 26 Suppl 4: D49–53.doi:10.1016/j.vaccine.2008.07.039. PMID 19230160.
- ^ Kimura H, Abiko C, Peng G, et al. (April 1997). "Interspecies transmission of influenza C virus between humans and pigs".Virus Research 48 (1): 71–9. doi:10.1016/S0168-1702(96)01427-X. PMID 9140195.
- ^ a b Matsuzaki Y, Sugawara K, Mizuta K, et al. (February 2002). "Antigenic and genetic characterization of influenza C viruses which caused two outbreaks in Yamagata City, Japan, in 1996 and 1998".Journal of Clinical Microbiology 40 (2): 422–9.doi:10.1128/JCM.40.2.422-429.2002. PMC 153379.PMID 11825952.
- ^ Lynch JP, Walsh EE (April 2007). "Influenza: evolving strategies in treatment and prevention". Semin Respir Crit Care Med 28 (2): 144–58. doi:10.1055/s-2007-976487. PMID 17458769.
- ^ a b c "Swine Influenza". Swine Diseases (Chest). Iowa State University College of Veterinary Medicine.
- ^ Ma W, Vincent AL, Gramer MR, et al. (December 2007). "Identification of H2N3 influenza A viruses from swine in the United States". Proceedings of the National Academy of Sciences of the United States of America 104 (52): 20949–54.doi:10.1073/pnas.0710286104. PMC 2409247.PMID 18093945.
- ^ Shin JY, Song MS, Lee EH, et al. (November 2006). "Isolation and characterization of novel H3N1 swine influenza viruses from pigs with respiratory diseases in Korea". Journal of Clinical Microbiology 44 (11): 3923–7. doi:10.1128/JCM.00904-06.PMC 1698339. PMID 16928961.
- ^ a b c d e f g Kothalawala H, Toussaint MJ, Gruys E (June 2006). "An overview of swine influenza". Vet Q 28 (2): 46–53.PMID 16841566.
- ^ Yassine HM, Al-Natour MQ, Lee CW, Saif YM (2007). "Interspecies and intraspecies transmission of triple reassortant H3N2 influenza A viruses". Virology Journal 4: 129.doi:10.1186/1743-422X-4-129. PMC 2228287.PMID 18045494.
- ^ "Swine influenza A (H1N1) infection in two children --- Southern California, March--April 2009". Morbidity and Mortality Weekly Report (Centers for Disease Control) 58 (Dispatch) (1–3). 22 April 2009.
- ^ "Interview With Tracey McNamara". Journal of Homeland Security, August 2002. Retrieved 2009-05-26.
- ^ Laura H. Kahn (2007-03-13). "Animals: The world's best (and cheapest) biosensors". Retrieved 2005-05-26.
- ^ a b c Knobler S, Mack A, Mahmoud A, Lemon S, ed. "1: The Story of Influenza". The Threat of Pandemic Influenza: Are We Ready? Workshop Summary (2005). Washington, D.C.: The National Academies Press. p. 75.
- ^ a b Olsen CW (May 2002). "The emergence of novel swine influenza viruses in North America". Virus Research 85 (2): 199–210. doi:10.1016/S0168-1702(02)00027-8. PMID 12034486.
- ^ Boffey, Philip M. (5 September 1976). "Soft evidence and hard sell". New York Times.
- ^ a b c d Taubenberger JK, Morens DM (2006). "1918 Influenza: the mother of all pandemics". Emerg Infect Dis 12 (1): 15–22.PMID 16494711.
- ^ "U.S. pork groups urge hog farmers to reduce flu risk". Reuters. 26 April 2009.
- ^ Heinen, P. (2003). "Swine influenza: a zoonosis". Veterinary Sciences Tomorrow: 1–11. Retrieved 2009-05-04.
- ^ Kay RM, Done SH, Paton DJ (August 1994). "Effect of sequential porcine reproductive and respiratory syndrome and swine influenza on the growth and performance of finishing pigs". Vet. Rec. 135 (9): 199–204. doi:10.1136/vr.135.9.199.PMID 7998380.
- ^ Vana G, Westover KM (June 2008). "Origin of the 1918 Spanish influenza virus: a comparative genomic analysis". Molecular Phylogenetics and Evolution 47 (3): 1100–10.doi:10.1016/j.ympev.2008.02.003. PMID 18353690.
- ^ Antonovics J, Hood ME, Baker CH (April 2006). "Molecular virology: was the 1918 flu avian in origin?". Nature 440 (7088): E9; discussion E9–10. doi:10.1038/nature04824.PMID 16641950.
- ^ Patterson KD, Pyle GF (1991). "The geography and mortality of the 1918 influenza pandemic". Bulletin of the History of Medicine 65(1): 4–21. PMID 2021692.
- ^ a b Gaydos JC, Top FH, Hodder RA, Russell PK (January 2006)."Swine influenza a outbreak, Fort Dix, New Jersey, 1976".Emerging Infectious Diseases 12 (1): 23–8. PMID 16494712.
- ^ Schmeck, Harold M. (March 25, 1976). "Ford Urges Flu Campaign To Inoculate Entire U.S.". The New York Times.
- ^ Richard E. Neustadt and Harvey V. Fineberg. (1978). The Swine Flu Affair: Decision-Making on a Slippery Disease. National Academies Press.
- ^ "The Last Great Swine Flu Epidemic", Salon.com, April 28, 2009.
- ^ Retailliau HF, Curtis AC, Storr G, Caesar G, Eddins DL, Hattwick MA (March 1980). "Illness after influenza vaccination reported through a nationwide surveillance system, 1976-1977".American Journal of Epidemiology 111 (3): 270–8.PMID 7361749.
- ^ "Historical National Population Estimates: July 1, 1900 to July 1, 1999". Washington D.C.: Population Division, U.S. Bureau of the Census. 2000-06-28. Retrieved 2009-08-21.
- ^ Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. (August 1979). "Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976--1977". American Journal of Epidemiology 110 (2): 105–23.PMID 463869.
- ^ a b c "General Questions and Answers on Guillain-Barr syndrome". Centers for Disease Control and Prevention. September 14, 2009.
- ^ Haber P, Sejvar J, Mikaeloff Y, DeStefano F (2009). "Vaccines and Guillain-Barré syndrome". Drug Safety 32 (4): 309–23.doi:10.2165/00002018-200932040-00005. PMID 19388722.
- ^ Kaplan JE, Katona P, Hurwitz ES, Schonberger LB (August 1982). "Guillain-Barré syndrome in the United States, 1979-1980 and 1980-1981. Lack of an association with influenza vaccination".JAMA 248 (6): 698–700. doi:10.1001/jama.248.6.698.PMID 7097920.
- ^ Vellozzi C, Burwen DR, Dobardzic A, Ball R, Walton K, Haber P (March 2009). "Safety of trivalent inactivated influenza vaccines in adults: Background for pandemic influenza vaccine safety monitoring". Vaccine 27 (15): 2114–2120.doi:10.1016/j.vaccine.2009.01.125. PMID 19356614.
- ^ "Last Year's (2009) H1N1 Flu Vaccine Was Safe, Study Finds". Wunderground.com. 2011-02-02. Retrieved 2011-05-22.
- ^ McKinney WP, Volkert P, Kaufman J (January 1990). "Fatal swine influenza pneumonia during late pregnancy". Archives of Internal Medicine 150 (1): 213–5. doi:10.1001/archinte.150.1.213.PMID 2153372.
- ^ Kimura K, Adlakha A, Simon PM (March 1998). "Fatal case of swine influenza virus in an immunocompetent host". Mayo Clinic Proceedings. Mayo Clinic 73 (3): 243–5. doi:10.4065/73.3.243.PMID 9511782.
- ^ "Key Facts About Swine Flu (CDC)". Cdc.gov. Retrieved 2009-05-07.
- ^ Wells DL, Hopfensperger DJ, Arden NH, et al. (1991). "Swine influenza virus infections. Transmission from ill pigs to humans at a Wisconsin agricultural fair and subsequent probable person-to-person transmission". JAMA 265 (4): 478–81.doi:10.1001/jama.265.4.478. PMID 1845913.
- ^ Stephanie Desmon (April 28, 2009). "Expert: Swine flu virus more complex than typically seen". Baltimore Sun.[dead link]
- ^ "Pork industry is blurring the science of swine flu - Short Sharp Science". New Scientist. Retrieved 2009-05-07.
- ^ "Swine flu: The predictable pandemic? - 29 April 2009". New Scientist. Retrieved 2009-05-07.
- ^ CDC Confirms Ties to Virus First Discovered in U.S. Pig Factories[dead link]
- ^ Video Segments 3,4,5 in Flu Factories: Tracing the Origins of the Swine Flu Pandemic[dead link]
- ^ Swine Flu Kept Stable Humans Untouched For 80 Years[dead link]
- ^ Triple Hybrid Mutant Pig-Bird-Human Crossbreed Virus[dead link]
- ^ North Carolina, 1998 Ground Zero[dead link]
- ^ "DA probes reported swine flu 'outbreak' in N. Ecija". Gmanews.tv. Retrieved 2009-04-25.
- ^ "Gov't declares hog cholera alert in Luzon". Gmanews.tv. Retrieved 2009-04-25.
- ^ "New mothers urged to get swine flu vaccine". BBC News. 2011-01-10. Retrieved 2011-01-20.
- ^ a b c d e "Influenza Factsheet". Center for Food Security and Public Health, Iowa State University.
- ^ Gilchrist MJ, Greko C, Wallinga DB, Beran GW, Riley DG, Thorne PS (February 2007). "The potential role of concentrated animal feeding operations in infectious disease epidemics and antibiotic resistance". Environmental Health Perspectives 115 (2): 313–6.doi:10.1289/ehp.8837. PMC 1817683. PMID 17384785.
- ^ Saenz RA, Hethcote HW, Gray GC (2006). "Confined animal feeding operations as amplifiers of influenza". Vector Borne and Zoonotic Diseases 6 (4): 338–46. doi:10.1089/vbz.2006.6.338.PMC 2042988. PMID 17187567.
- ^ Vicente J, León-Vizcaíno L, Gortázar C, José Cubero M, González M, Martín-Atance P (July 2002). "Antibodies to selected viral and bacterial pathogens in European wild boars from southcentral Spain". Journal of Wildlife Diseases 38 (3): 649–52.PMID 12238391.
- ^ a b Gray GC, Kayali G (April 2009). "Facing pandemic influenza threats: the importance of including poultry and swine workers in preparedness plans". Poultry Science 88 (4): 880–4.doi:10.3382/ps.2008-00335. PMID 19276439.
- ^ Gray GC, Trampel DW, Roth JA (May 2007). "Pandemic influenza planning: shouldn't swine and poultry workers be included?".Vaccine 25 (22): 4376–81. doi:10.1016/j.vaccine.2007.03.036.PMC 1939697. PMID 17459539.
- ^ Gray GC, McCarthy T, Capuano AW, Setterquist SF, Olsen CW, Alavanja MC (December 2007). "Swine workers and swine influenza virus infections". Emerging Infectious Diseases 13(12): 1871–8. PMC 2876739. PMID 18258038.
- ^ Myers KP, Olsen CW, Setterquist SF, et al. (January 2006). "Are swine workers in the United States at increased risk of infection with zoonotic influenza virus?". Clinical Infectious Diseases 42 (1): 14–20. doi:10.1086/498977. PMC 1673212.PMID 16323086.
- ^ a b Thacker E, Janke B (February 2008). "Swine influenza virus: zoonotic potential and vaccination strategies for the control of avian and swine influenzas". J. Infect. Dis. 197 Suppl 1: S19–24.doi:10.1086/524988. PMID 18269323.
- ^ Yu H, Hua RH, Zhang Q, et al. (March 2008). "Genetic evolution of swine influenza A (H3N2) viruses in China from 1970 to 2006".Journal of Clinical Microbiology 46 (3): 1067–75.doi:10.1128/JCM.01257-07. PMC 2268354.PMID 18199784.
- ^ Lindstrom SE, Cox NJ, Klimov A (October 2004). "Genetic analysis of human H2N2 and early H3N2 influenza viruses, 1957-1972: evidence for genetic divergence and multiple reassortment events". Virology 328 (1): 101–19.doi:10.1016/j.virol.2004.06.009. PMID 15380362.
- ^ World Health Organization (28 October 2005). "H5N1 avian influenza: timeline" (PDF).
- ^ "Indonesian pigs have avian flu virus; bird cases double in China". University of Minnesota: Center for Infectious Disease Research & Policy. 27 May 2005. Retrieved 2009-04-26.
- ^ "H5N1 virus may be adapting to pigs in Indonesia". University of Minnesota: Center for Infectious Disease Research & Policy. 31 March 2009. Retrieved 2009-04-26. report on pigs as carriers.
- ^ "Centers for Disease Control and Prevention > Key Facts about Swine Influenza (Swine Flu)". Retrieved April 27, 2009.
- ^ a b c d Myers KP, Olsen CW, Gray GC (April 2007). "Cases of swine influenza in humans: a review of the literature". Clinical Infectious Diseases 44 (8): 1084–8. doi:10.1086/512813.PMC 1973337. PMID 17366454.
- ^ " Symptoms of H1N1 (Swine Flu) ". YouTube. 2009-04-28. Retrieved 2011-05-22.
- ^ "Swine Flu and You". CDC. 2009-04-26. Retrieved 2009-04-26.
- ^ a b Centers for Disease Control and Prevention (April 27, 2009)."CDC Health Update: Swine Influenza A (H1N1) Update: New Interim Recommendations and Guidance for Health Directors about Strategic National Stockpile Materiel". Health Alert Network. Retrieved April 27, 2009.
- ^ "Study: Swine flu resembles feared 1918 flu". MSNBC. 2009-07-13. Retrieved 2011-05-22.
- ^ "Swine flu can damage kidneys, doctors find". Reuters. April 14, 2010. Retrieved April 17, 2010.
- ^ "CDC H1N1 Flu | Interim Guidance on Specimen Collection, Processing, and Testing for Patients with Suspected Novel Influenza A (H1N1) (Swine Flu) Virus Infection". Cdc.gov. 2009-05-13. Retrieved 2011-05-22.
- ^ "Micronics Acquires License to Biosearch Technologies’ Nucleic Acid Assay Chemistries". Biosearchtech.com. 2009-10-28. Retrieved 2011-05-22.
- ^ "Swine flu virus turns endemic". National Hog Farmer. 15 September 2007.
- ^ "Swine". Custom Vaccines. Novartis.[dead link]
- ^ Gramer MR, Lee JH, Choi YK, Goyal SM, Joo HS (July 2007). "Serologic and genetic characterization of North American H3N2 swine influenza A viruses". Canadian Journal of Veterinary Research = Revue Canadienne De Recherche Vétérinaire 71 (3): 201–6. PMC 1899866. PMID 17695595.
- ^ "Swine flu: The predictable pandemic?". 2009-04-29.
- ^ Ramirez A, Capuano AW, Wellman DA, Lesher KA, Setterquist SF, Gray GC (June 2006). "Preventing zoonotic influenza virus infection". Emerging Infect. Dis. 12 (6): 996–1000.PMC 1673213. PMID 16707061.
- ^ a b "Q & A: Key facts about swine influenza (swine flu) – Spread of Swine Flu". Centers for Disease Control and Prevention. 24 April 2009. Retrieved 2009-04-26.
- ^ a b "CDC H1N1 Flu | H1N1 Flu and You". Cdc.gov. Retrieved 2011-05-22.
- ^ "Q & A: Key facts about swine influenza (swine flu) – Diagnosis". Centers for Disease Control and Prevention. 24 April 2009. Retrieved 2009-04-26.
- ^ "CDC - Influenza (Flu) | Swine Influenza (Flu) Investigation". Cdc.gov. Retrieved 2009-04-27.
- ^ "Chlorine Bleach: Helping to Manage the Flu Risk". Water Quality & Health Council. April 2009. Retrieved 2009-05-12.
- ^ "Self protection measures". LHC. Retrieved 2009-10-15.
- ^ "FDA Approves Vaccines for 2009 H1N1 Influenza Virus". FDA. Retrieved 2009-10-15.
- ^ "NIH studies on Swine flu vaccine". NIH. Retrieved 2009-10-15.[dead link][dead link]
- ^ http://www.who.int/csr/disease/swineflu/faq/en/index.html
- ^ "Antiviral Drugs and Swine Influenza". Centers for Disease Control. Retrieved 2009-04-27.
- ^ "FDA Authorizes Emergency Use of Influenza Medicines, Diagnostic Test in Response to Swine Flu Outbreak in Humans.FDA News, April 27, 2009". Fda.gov. 2009-04-27. Retrieved 2009-05-07.
[edit]Further reading
- Alexander DJ (October 1982). "Ecological aspects of influenza A viruses in animals and their relationship to human influenza: a review". Journal of the Royal Society of Medicine 75 (10): 799–811.PMC 1438138. PMID 6752410.
- Hampson AW, Mackenzie JS (November 2006). "The influenza viruses". The Medical Journal of Australia 185 (10 Suppl): S39–43.PMID 17115950.
- Lipatov AS, Govorkova EA, Webby RJ, et al. (September 2004). "Influenza: emergence and control". Journal of Virology 78 (17): 8951–9. doi:10.1128/JVI.78.17.8951-8959.2004. PMC 506949.PMID 15308692.
- Van Reeth K (2007). "Avian and swine influenza viruses: our current understanding of the zoonotic risk". Veterinary Research 38 (2): 243–60. doi:10.1051/vetres:2006062. PMID 17257572.
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y (March 1992). "Evolution and ecology of influenza A viruses".Microbiological Reviews 56 (1): 152–79. PMC 372859.PMID 1579108.
- Winkler WG (October 1970). "Influenza in animals: its possible public health significance". Journal of Wildlife Diseases 6 (4): 239–42; discussion 247–8. PMID 16512120.
[edit]External links
| Wikimedia Commons has media related to: Swine flu |
| Wikinews has related news:Swine flu |
- Swine influenza: Slideshow
- UK National Pandemic Flu Service
- Official UK government information on swine flu from Directgov
- Official swine flu advice and latest information from the UK National Health Service
- 8 minute video answering common questions about the subject on fora.tv
- Swine flu charts and maps Numeric analysis and approximation of current active cases
- Worried about swine flu? Then you should be terrified about the regular flu.
- Centers for Disease Control and Prevention (CDC) - Swine Flu
- Pandemic Flu US Government Site
- World Health Organization (WHO): Swine influenza
- Medical Encyclopedia Medline Plus: Swine Flu
- Swine Flu News and Updates From Around the World
- Swine Flu Tracker
- Health-EU portal EU response to influenza
- European Commission - Public Health EU coordination on Pandemic (H1N1) 2009
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||






Dr. Imoloa has really made me so much believe in him by getting me cured with his herbal treatment. i really appreciate you Dr.imoloa for bringing back happiness to my life again. thank you so much,friends join me to thank him for what he has actually done for me i pray to you all for a good life and good health, and most especially to you Dr. imoloa Thanks
ReplyDeleteI have been suffering from (HERPES SIMPLEX VIRUS) disease for the past four years and had constant pain, especially in my knees. During the first year,I had faith in God that i would be healed someday.This disease started circulating all over my body and i have been taking treatment from my doctors, few months ago i came on search on the internet if i could get any information concerning the cure of this disease, on my search i saw a testimony of someone who has been healed from (HERPES SIMPLEX VIRUS) by this Man Dr imoloa and she drop the email address of this man and advise we should contact him for any sickness that he would be of help, so i wrote to Dr. imoloa telling him about my (HERPES Virus) well after all the procedures and remedy given to me by this man few weeks later i started experiencing changes all over me. I am now here to testify that i am no longer a herpes patient, I have experience a total transformation in my life,for all herpes patients get your herbal medicine to cure your sickness. And there has being rapid improvement in my health, I no longer feel pains and I wake up each morning feeling revived. So friends my advise is if you have such sickness or any other at all,you can contact him on drimolaherbalmademedicine@gmail.com, you can still reach him on whatssap- +2347081986098
CANCER
EPILEPSY.
GENPILENCIN.
HIV AIDS.
DIABETICS
STROKE.
BREAST ENLARGEMENT...PENIS ENLARGEMENT, LUPUS DISEASE, BREAST CANCER, BONE CANCER, FEVER, DIARRHOEA, ARTHRITIS, DRY COUGH, MUSCLE ACHES, FATIGUE, H.P.V TYPE 1 TYPE 2 TYPE 3 AND TYPE 4. TYPE HUMAN PAPAILOMA VIRUS HERPES. SYPHILIS. HEPATITIS A B and C.